![]()
Improper dihedrals are used to restrict the geometry of molecules and as such need not have a simple relation to conventional chemical bonding. DL_POLY_2 makes no distinction between dihedral angle functions and improper dihedrals (both are calculated by the same subroutines) and all the comments made in the preceeding section apply.
An important example of the use of the improper dihedral is to
conserve the structure of chiral centres in molecules modelled by united-atom centres. For
example ![]() -amino acids such as alanine (CH
-amino acids such as alanine (CH![]() CH(NH
CH(NH![]() )COOH), in which it is common to represent the CH
)COOH), in which it is common to represent the CH![]() and CH
groups as single centres. Conservation of the chirality of the
and CH
groups as single centres. Conservation of the chirality of the ![]() carbon is achieved by defining a
harmonic improper dihedral angle potential with an equilibrium angle of
35.264
carbon is achieved by defining a
harmonic improper dihedral angle potential with an equilibrium angle of
35.264![]() .
The angle is defined by vectors
.
The angle is defined by vectors ![]() ,
, ![]() and
and ![]() , where the atoms 1,2,3 and 4 are shown in the
following figure. The figure defines the D and L enantiomers consistent with the
international (IUPAC) convention. When defining the dihedral, the atom
indices are entered in DL_POLY_2 in the order 1-2-3-4.
, where the atoms 1,2,3 and 4 are shown in the
following figure. The figure defines the D and L enantiomers consistent with the
international (IUPAC) convention. When defining the dihedral, the atom
indices are entered in DL_POLY_2 in the order 1-2-3-4.
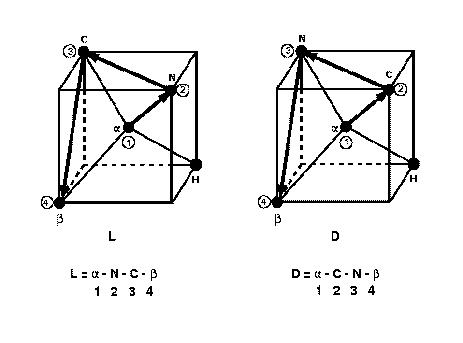
The L and D enantiomers and defining vectors
In DL_POLY_2 improper dihedral forces are handled by the routine DIHFRC.
![]()